I agree to and consent to receive news, updates, and other communications by way of commercial electronic messages (including email) from The Uranium Producers of America. I understand I may withdraw consent at any time by clicking the unsubscribe link contained in all emails from The Uranium Producers of America.
In situ recovery
URANIUM IN SITU RECOVERY TECHNOLOGY
Many U.S. uranium deposits can be recovered commercially by modern, low cost in situ recovery (ISR) technology. As described below, this type of mineral recovery involves the circulation of groundwater with bubbled oxygen and club-soda-like mixture through a series of injection and extraction wells until the uranium in the sand of the aquifer has been depleted.
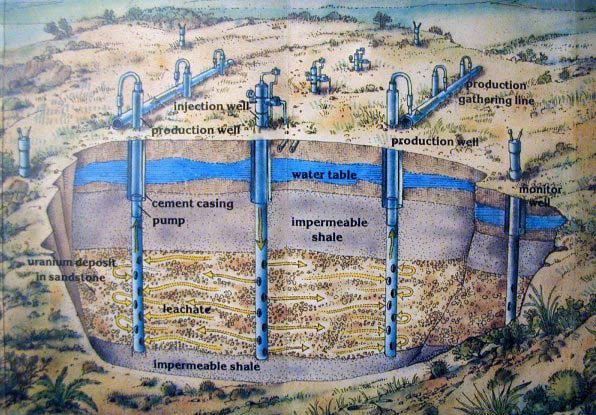
Modern ISR involves the circulation of naturally occurring and benign groundwater, fortified with oxygen, through a uranium ore body. This natural water plus oxygen is pumped into injection wells, through the uranium ore body, where the uranium in the host sandstone is oxidized and solubilized - continuing through the sandstone to the extraction wells, where the uranium-bearing groundwater is pumped to the surface. This water proceeds to a ion exchange unit (like a big water-softener) for uranium removal then is pumped back to the wellfield for re-fortification with oxygen, down into the injection wells, and again re-circulated through the ore body. This recirculation of the same groundwater continues over and over, until the uranium in the sandstone is depleted.
The water found in and adjacent to uranium ore body is naturally contaminated and unsuitable for drinking, even if it is surrounded by a drinking water aquifer. Uranium decays into radium and radon gas, and other “daughter” products. Over hundreds of thousands, or millions, of years, these decay products build up and contaminate the water in and near the ore body. For example, the U.S. EPA has a proposed maximum level for radon gas of 300 pCi/L in drinking water. In a uranium orebody, it is common for radon gas to exceed 1,000,000 pCi/L, which is 3,300 times the drinking water standard. Water from a uranium ore body is naturally contaminated and cannot be used for any activity (e.g., irrigation), let alone as a source of drinking water.
Oxygen is added to the naturally contaminated groundwater at the ore body, and that water is continuously re-circulated until most of the uranium is recovered. The technology used to take the uranium out of the water is the same as that used in home-based water softeners. Waste in ISR is only a tiny fraction of that from a conventional mine, so tailing piles are not needed at the site, and the footprint of ISR facilities is far smaller than that for a conventional mine operation.
ISR is highly regulated at every phase of operation, and monitor wells surrounding the mine site are required, ensuring protection of the surrounding aquifer. In ISR the aquifer must be restored to baseline conditions.
Uranium ISR is not new; it has been safely used for more than thirty years. Projects using ISR occur in Nebraska, Texas, and Wyoming, and in other countries around the world, including Kazakhstan and Australia.
The Ore Body
The original source of uranium is igneous (volcanic) rock, which includes all of the earth at one time or another and which makes uranium ubiquitous. Uranium is commonly found in water because it oxidized and its oxidized form is very water soluble. Conversely, when the soluble uranium comes into contact with a reducing environment (e.g., sulfides such as pyrite or hydrogen sulfide, and organic material such as coal, oil or gas), it falls from solution.
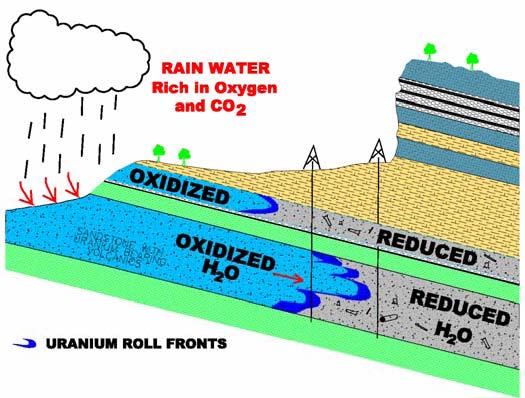 Uranium enters an aquifer when it becomes dissolved in
Uranium enters an aquifer when it becomes dissolved in
oxygenated waters, such as rainwater. The oxygenated water percolates downward from the surface, through uranium bearing sediments, such as rock interbedded with volcanic ash. As the uranium is dissolved into the water, it is carried even further through the porous rock and deeper into the aquifer. Commercial grade ore deposits accumulate over millions of years, as huge amounts of weakly oxidized groundwater containing the dissolved uranium pass a reducing interface in the sandstone rock, where the uranium accumulates.
These uranium ore deposits must meet certain criteria to allow recovery with the ISR process:
Multiple uranium ore horizons, 30 to 150 feet wide, are identified through drilling of exploration boreholes. The exact location of the ore is then mapped, showing its length, width, and depth below surface. Injection and extraction (production) wells are drilled, cased, cemented, tested, and completed in the ore zones. These wells are placed in patterns, coupling injectors with extractors, and spanning the ore body. These patterns cause the oxygenated water that is injected at the surface to be pulled through and across the ore, taking the ore with it when it is then pumped from the extraction wells back up to the surface. This technology was first developed and refined by the oil industry, which uses the same technique in water floods or other secondary and tertiary oil recovery projects.
- The ore deposit must be located in a water-saturated zone.
- The deposit must have adequate rock permeability.
- The deposit must be easily solubilized with oxygen.
Monitor wells completely surround the wellfield patterns, and are sampled regularly (sometimes as often as twice weekly) to ensure that all recovery solutions remain contained within the mineralized area. For this same reason, monitor wells are placed in sands immediately above and below the ore zone, as required.
Wellfield and Production of Uranium
Conventional uranium mills, which treat rock taken from open pit or underground mines, mostly use sulfuric acid to dissolve uranium from the crushed rock. That solution is called "lixiviant" or "leachate". In early tests of ISR, sulfuric acid solutions or ammonium carbonate-hydrogen peroxide solutions were tried (especially the ammonium carbonate lixiviant). While these leach solutions readily removed the uranium mineral from the host sand, they caused problems in the post-production groundwater restoration phase, and were quickly replaced with a more neutral, environmentally friendly solution.
Today, ISR operations in the U.S. use a sodium bicarbonate-carbonate system, a solution similar to baking soda or club soda. This system closely resembles the chemistry of native groundwater. Gaseous oxygen is added to the water to mobilize the uranium, which then is “washed” from the sand grains by the carbonate present in the water, basically mimicking (in reverse order) the natural process of uranium emplacement. This water is then pumped from the ground to the surface plant for removal of the uranium.
The actual ISR process begins when groundwater is pumped from the extraction/ production wells. The water flows through the uranium extraction plant described below. After the groundwater is circulated through the plant, oxygen is added to the water. This "fortified" water is injected into the ore horizon through injection wells. As the oxygenated water passes through the porous sandstone, it oxidizes and solubilizes the uranium, which generally occurs as a thin precipitate covering the sand grains of sedimentary rock. This water is pulled to the extraction wells and pumped to the surface plant for uranium removal. The cycle is repeated, and the groundwater is recirculated over and over.
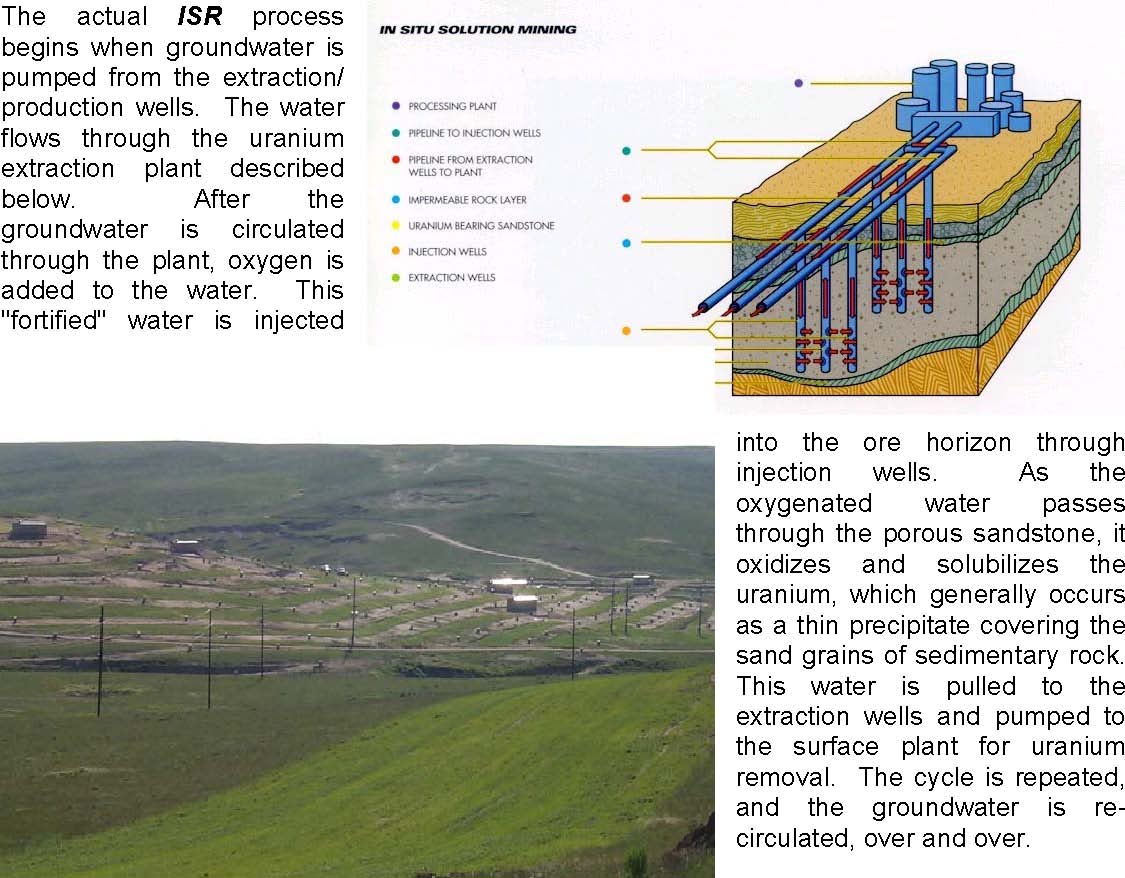
Generally, ISR is a closed water system, circulating the same water over and over again, except that approximately 1% less water is injected than was extracted. This creates a "pressure sink" in the wellfields, causing fluids in the area to naturally flow into the wellfield, thereby helping to contain the lixiviant solutions.
Surface Facilities: Ion Exchange, Elution, Precipitation, and Drying
ION EXCHANGE. The surface plant for an ISR operation is essentially a large water softener, very similar to those used in homes. "Hard water" in the home is caused by high levels of the chemical ions of calcium and magnesium. A home water softener contains plastic "resin" beads held in a tank (itself generally plastic or fiberglass). As water flows through the tank and across these resin beads, the chemical ions of calcium and magnesium are "exchanged" out of the water and onto the resin. The other half of the "exchange" is sodium ions transferring off the resin and into the water. This is called "softening" of water, and the term "ion exchange" is used for this general process.
Highly concentrated salt water (or brine) will cause this ion exchange to reverse direction. During the "regeneration" cycle of the home water softener, a highly concentrated salt (NaCl) solution is pumped over the resin, causing the calcium and magnesium to "exchange" off the resin and into the salt water (opposite of what it did in the "softening" process), while sodium transfers back onto the resin, thus "regenerating" the resin and preparing it again for "softening" of water.
The same type of "ion exchange" will transfer uranium out of water and onto resin beads. An ISR facility is composed mostly of large tanks that hold "resin" and "regenerating" water, and pumps to move the water. A home water softener contains "cation" resin because it involves the ion exchange of the positively charged ions (cations): calcium, magnesium, and sodium. Since uranium in solution is a negatively charged ion (called anion), the tanks at a recovery plant hold "anion" resin beads.
Other than this, the ion exchange process for uranium ISR is exactly the same as for a home water softener. The water pumped from the ground contains uranium in solution, passes over the anion resin, and the uranium transfers onto the plastic resin beads. At the same time, chloride or bicarbonate (both negative ions) transfers off the resin and into the water. The ion exchange is then complete, and the groundwater has very little uranium left in it. Once the water leaves the resin tank, it is re-fortified with oxygen and re-injected into the ground. The process is repeated again and again, until the uranium level drops too low to continue the cycle.
REMOTE ION EXCHANGE (“RIX”) Smaller ore US deposits may not support a large central plant and but rather would call for RIX. In an RIX system, each orebody is still mined with its own native groundwater, and the recovery process is the same, except that the surface facility is off-site. The resin, once loaded with uranium, is transferred out of the ion exchange column, drained, and trucked via trailer to a central plant for removal of the captured uranium. The clean ion exchange resin is then transferred back to the RIX in the same way.
Each RIX is a self-contained, stand-alone unit that recovers uranium in pressurized down flow ion exchange columns. A pressurized system keeps the uranium solution contained within a closed loop that eliminates the potential for release of radon gas to the atmosphere. (Radon gas is a natural byproduct of uranium decay, and is always present along with the parent uranium.) The entire unit is curbed to provide containment from spills or leakage.
The RIX system allows development of only one central process plant to be used for multiple orebodies. This lowers capital outlay and allows recovery from smaller ore deposits.
ELUTION. The "regeneration" phase for ISR resin is called "elution", and the salt water used to regenerate the resin is called "eluent". Just as for the home water softener, highly concentrated salt water brine is used for regeneration. In the case of ISR however, some sodium bicarbonate-carbonate solution (baking soda and club soda) is mixed with the brine. This brine water is then pumped over the resin, and a reverse ion exchange occurs, just as it does in a home water softener during the regenerating phase. The elution process causes uranium to be concentrated in the saltwater brine or "eluent". For a home water softener, the brine is used only once and then is sent down the drain, but in the ISR process, the eluent is recycled and reused after the uranium is precipitated from it.
PRECIPITATION. Precipitation of the uranium from the eluent, or saltwater brine, is also a relatively simple and uncomplicated process. At this point, the uranium is actually combined with carbonate, known chemically as a "complex". A small amount of hydrochloric acid, also known as muriatic acid when used in home swimming pools, is added to the brine or eluant. This causes the carbonate to break apart into carbon dioxide and oxygen, breaking the uranium-carbonate "complex". The uranium is now free and can be chemically "reduced" to cause precipitation. Chemical reduction is simply repeating the natural process that formed the ore body in the first place. Alternatively, the uranium can be oxidized even further, which forms a precipitate of crystals. Since "crystals" are relatively easy to filter from water, oxidation is the preferred method of precipitation. Hydrogen peroxide, just like you buy in the drugstore, is used for this step. The brine is then refortified with salt and carbonate in preparation for another cycle. Impurities, such as sulfate, can build up in the brine and decrease efficiency of the regeneration process, so some of the brine is replaced with fresh solution. The used or "spent" eluant is considered a waste product, and is discharged to a holding pond.
PRODUCT DRYING AND PACKAGING. The precipitated and filtered uranium, also called uranium "slurry", is washed with a small amount of fresh water while in the filter press, and then dewatered. This wash water, which contains some soluble uranium, is returned to the elution and precipitation circuit. The resulting moist uranium slurry (or "yellowcake") may be dried prior to packaging for shipment. If the uranium is to be dried, it is pumped into a zero-emission vacuum dryer. The water liberated as steam during the drying process is condensed, and recycled to the precipitation circuit or discharged to a waste pond. The dried yellowcake is placed in 55-gallon steel drums for shipment.
Groundwater Restoration
When production terminates, the water quality of the affected aquifer must be restored to levels defined in regulatory standards. The same injection, and production wells, and surface facilities are used for groundwater restoration. Restoration reduces the effects of ISR by removing injected chemicals or immobilizing substances produced by the process.
Several restoration methods are currently used. The most direct and widely practiced technique is that of groundwater sweeping, which is simply pumping waters out of the well field area. As pumping continues, groundwater beyond the mineralized area flows in to replace the fluid that was pumped out. The extent of lixiviant recovery and removal of undesired ions in the aquifer improves with increased groundwater extraction, and the process continues until predetermined concentrations of constituents are attained.
A second restoration technique involves surface treatment of recycled groundwater before it is reinjected into the wells. Recycling uses some sort of filtration or distillation process such as reverse osmosis concentration to remove the desired constituents from water and consumes less groundwater than sweeping. Reverse osmosis is a well established water treatment process whereby the majority of dissolved "ions" are separated from the wastewater, and concentrated into a smaller volume of briny wastewater. After processing by reverse osmosis, pure water is then returned to the affected aquifer through injection wells. This reinjection of very pure water results in a large increment of water quality improvement in a short time period.
Wellfield Reclamation
Prior to surface reclamation, well bores are filled with cement to prevent groundwater migration between water-bearing formations. Casings are then cut off below the ground surface, and the resulting excavation backfilled. Surface soil may require decontamination to defined limits. Contaminated surface soil is considered a by-product material of the ISR process, and disposed of at a licensed site. Any structures remaining after license termination must be decontaminated to regulatory limits allowing unrestricted use. The ground surface must also be re-vegetated. All reclamation work is performed according to standards set by regulatory agencies, and is overseen by those agencies.
Waste Management ISR operations produce small amounts of both solid wastes, and liquid effluents.
No solid waste is disposed of permanently at ISR locations. Liquid wastes from the wellfield, process circuit, and aquifer restoration are often injected into deep Waste Disposal Wells (e.g., in Nebraska, Texas, Wyoming). ISR disposal wells are not classified as “hazardous”; as compared to the hundreds of “hazardous” disposal wells are used in the U.S. As with all phases of ISR, work is overseen by regulatory agencies, and follows standards established by them.

